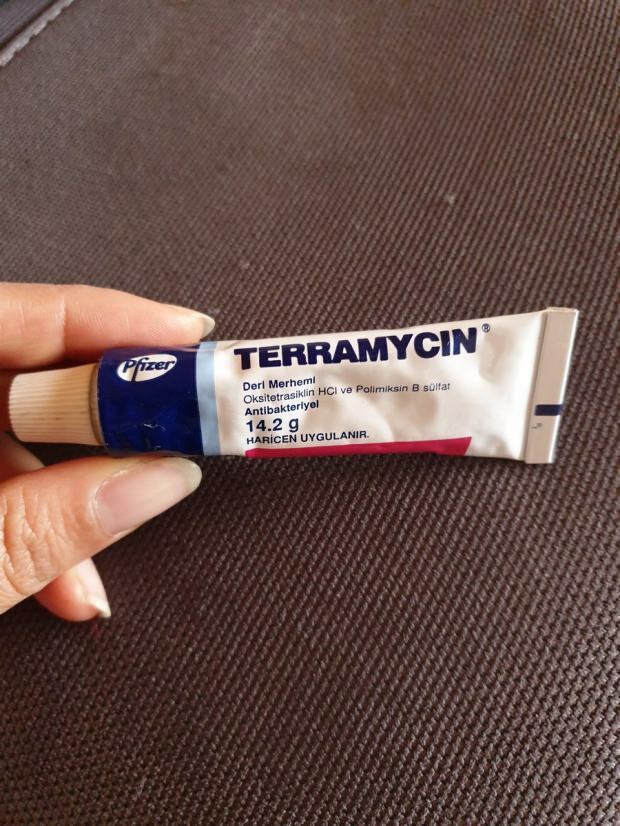
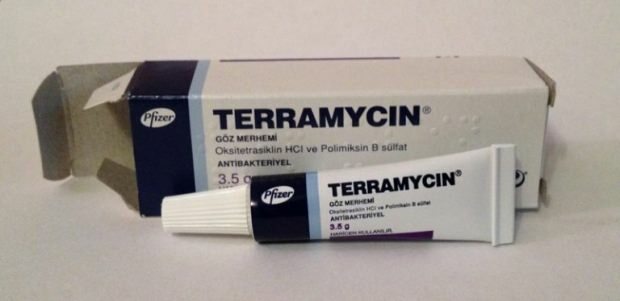
Террамицин (терамицин) крем, известный в течение многих лет и всегда в аптечных сумках, снова стал знаменитым. Вы должны использовать крем Terramycin (Teramycin), который является очень экономически доступным, для исследования и заживления ран. Так каковы преимущества крема Terramycin (Teramycin) для кожи? Как использовать крем террамицин (терамицин)? Сколько стоит крем террамицин (терамицин)? Вы можете найти все подробности в нашей статье.
Террамицин (продается в аптеках много лет)ты терамису) крем; Это крем для кожи, который устраняет многие проблемы в теле от отеков до покраснения и тратит на это немного времени. Этот крем быстро справляется с такими проблемами, как прыщи, воспаление, раны, ожоги, бактериальные инфекции, покраснение тела, и быстро лечит такие раны, как верхний слой кожи, волдыри или агломерацию. Terramycin (Teramycin) крем, который лечит раны после небольшого удара в результате незаметных условий, недавно использовался при воспаленных прыщах и фурункулах. Люди, которые испытывают какие-либо из этих проблем, должны использовать крем, который используется в очень короткое время.
Терамициновый крем, активный ингредиент которого содержит гидрохлорид окситетрациклина и сульфат полимиксина B, а также жидкий вазелин и белый вазелин, содержит два вспомогательных вещества. Крем терамицин, который имеет мягкий желтый однородный цвет, относится к группе антибиотиков, применяемых местно.
Вы можете использовать его, когда столкнетесь с одной из следующих проблем;
— При лечении больших нарывов,
— При воспалениях кожи и воспалительных состояниях,
— При лечении трещин губ,
— Лечение герпеса,
— Лечение ран и царапин на коже,
— При лечении укусов насекомых и пчел,
— Лечение угревой сыпи,
— При лечении ран на ухе,
— Может использоваться при лечении ран при многих инфекциях.
Как использовать мазь Террамицин?
Убедившись, что область, на которую вы будете наносить крем Террамицин, является чистой, нанесите крем на область кончиками пальцев. Затем накройте его марлей, чтобы сохранить гигиену.
Вы можете использовать этот крем, которого достаточно наносить дважды в день, пока рана не заживет.
КАКАЯ ЦЕНА ТЕРРАМИЦИНА НЕФТИ?
Цена на крем террамицин (терамицин) по состоянию на март 2020 года Это 19,33 TL.
Terramycin with Polymyxin B Prescribing Information
Package insert / product label
Generic name: oxytetracycline hydrochloride and polymyxin B sulfate
Dosage form: Ophthalmic Ointment
Drug class: Ophthalmic anti-infectives
Medically reviewed by Drugs.com. Last updated on Mar 24, 2023.
On This Page
- Description
- Indications and Usage
- Contraindications
- Precautions
- Patient Counseling Information
- Adverse Reactions/Side Effects
- Dosage and Administration
- How Supplied/Storage and Handling
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Terramycin® and other antibacterial drugs, Terramycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Terramycin with Polymyxin B Description
Each gram of sterile ointment contains oxytetracycline HCl equivalent to 5 mg oxytetracycline, 10,000 units of polymyxin B sulfate, white petrolatum, and liquid petrolatum.
ACTIONS
Terramycin is a widely used antibiotic with clinically proved activity against gram-positive and gram-negative bacteria, rickettsiae, spirochetes, large viruses, and certain protozoa.
Polymyxin B Sulfate, one of a group of related antibiotics derived from Bacillus polymyxa, is rapidly bactericidal. This action is exclusively against gram-negative organisms. It is particularly effective against Pseudomonas aeruginosa (B. pyocyaneus), and Koch-Weeks bacillus, frequently found in local infections of the eye.
There is thus made available a particularly effective antimicrobial combination of the broad-spectrum antibiotic Terramycin as well as polymyxin B sulfate against primarily causative or secondarily infecting organisms.
Indications and Usage for Terramycin with Polymyxin B
The sterile preparation, Terramycin with Polymyxin B Sulfate Ophthalmic Ointment, is indicated for the treatment of superficial ocular infections involving the conjunctiva and/or cornea caused by Terramycin with Polymyxin B Sulfate-susceptible organisms.
It may be administered topically alone, or as an adjunct to systemic therapy.
It is effective in infections caused by susceptible strains of staphylococci, streptococci, pneumococci, Hemophilus influenzae, Pseudomonas aeruginosa, Koch-Weeks bacillus, and Proteus.
To reduce the development of drug-resistant bacteria and maintain effectiveness of Terramycin and other antibacterial drugs, Terramycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Contraindications
This drug is contraindicated in individuals who have shown hypersensitivity to any of its components.
Precautions
As with all antibiotic preparations, use of this drug may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, the antibiotic should be discontinued and appropriate specific therapy should be instituted.
Prescribing Terramycin in the absence of proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information For Patients
Patients should be counseled that antibacterial drugs including Terramycin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Terramycin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Terramycin or other antibacterial drugs in the future.
Adverse Reactions/Side Effects
Terramycin with Polymyxin B Sulfate Ophthalmic Ointment is well tolerated by the epithelial membranes and other tissues of the eye. Allergic or inflammatory reactions due to individual hypersensitivity are rare.
Terramycin with Polymyxin B Dosage and Administration
Approximately ½ inch of the ointment is squeezed from the tube onto the lower lid of the affected eye two to four times daily.
The patient should be instructed to avoid contamination of the tip of the tube when applying the ointment.
How is Terramycin with Polymyxin B supplied
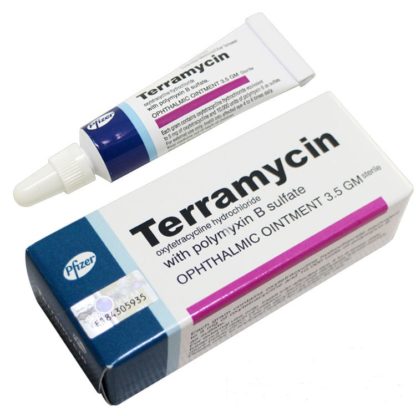
Terramycin with Polymyxin B Sulfate Ophthalmic Ointment is supplied in 1/8 oz (3.5 g) tubes (NDC 0049-0801-08).
Rx only
LAB-0193-3.0
| TERRAMYCIN oxytetracycline hydrochloride and polymyxin b sulfate ointment |
|||||||||||
|
|||||||||||
|
|||||||||||
|
|||||||||||
|
|||||||||||
Medical Disclaimer
Лекарственная форма
|
|
Террамицин LA |
Раствор для инъекций 200 мг рег. 840-3-15.17-3960№ПВИ-3-2.7/02134 |
Форма выпуска, состав и упаковка
Международное непатентованное или химическое наименование:
окситетрациклин
Держатель регистрационного удостоверения:
«Zoetis Inc.», 100 Campus Drive, Florham Park, New Jersey, 07932, США
Разработчик:
«Zoetis Inc.», 100 Campus Drive, Florham Park, New Jersey, 07932, США
Производитель:
«Zoetis Manufacturing & Research Spain, S.L.», Ctra. De Camprodon, s/n., Finca La Riba, Vall de Bianya, 17813 Gerona, Испания
«Laboratorios Pfizer Ltda», Av. Tancredo de Ameida Neves, 1, 555-Guarulhos-Бразилия-CEP.: 07112-070
Лекарственная форма:
раствор для инъекций
Качественный состав и количественный состав действующих веществ и качественный состав вспомогательных веществ:
в 1 мл в качестве действующего вещества окситетрациклина дигидрат — 200 мг, а в качестве вспомогательных веществ: 2-пирролидон — 400 мг, поливинил-пиролидон К17 — 50 мг, оксид магния -17,157 мг, моноэтаноламин — 2,4 мг и воду для инъекций — до 1 мл
Дозировка:
200 мг
Количество в потребительской упаковке:
по 100 мл во флаконах
Фармакотерапевтическая группа; код АТХ, рекомендованной ВОЗ:
Антибактериальные средства
Показания к применению препарата Террамицин LA
Применяют крупному рогатому скоту и свиньям для лечения болезней бактериальной этиологии, в том числе пастереллеза, риккетсиоза, пневмонии, некробактериоза и атрофического ринита у свиней
Побочные эффекты
При назначении в соответствии с инструкцией побочных явлений и осложнений, как правило, не отмечается. Возможно появление местной реакции в виде зуда, эритемы, отека.
Противопоказания к применению препарата Террамицин LA
Не следует применять препарат одновременно с кортикостероидами и эстрогенами, а также с бактерицидными препаратами. Убой животных на мясо разрешается не ранее, чем через 28 суток после последнего применения препарата.
Условия хранения Террамицин LA
Хранят в закрытой упаковке производителя, в защищенном от прямых солнечных лучей месте, отдельно от продуктов питания и кормов при температуре от 3 °С до 30 °С. После вскрытия флакона лекарственный препарат можно использовать в течение 28 дней.
Террамицин LA отзывы
Помогите другим с выбором, оставьте отзыв об Террамицин LA
Оставить отзыв
- Описание
- Детали
- Отзывы (0)
Описание
Глазная мазь для лечения воспалительных заболеваний глаз.
Каждый грамм мази содержит окситетрациклин гидрохлорид, эквивалентный 5 мг окситетрациклина и 10000 единиц сульфата полимиксина В.
Показания к применению: инфекционно-воспалительные заболевания глаз ( хламидиоз, микоплазмоз, стафилококковая и стрептококковая инфекция), профилактика развития вторичных инфекций при вирусных заболеваниях.
Применение: закладывать в глаза (под нижнее веко) 2-4 раза в день.
Импортировано в Таиланд.
Только зарегистрированные клиенты, купившие этот товар, могут публиковать отзывы.

Террамицин офтальмологическая мазь для кошек или собак
1 заказ
571 руб.
Описание
Терамицин офтальмологическая мазь с полимиксином B сульфат показан у собак и кошек с поверхностными глазными инфекциями, такими как конъюцтивит, кератит, розовый глаз, язва роговицы, блефарит и бактериальные противовоспалительные состояния, которые могут возникнуть вторично для других инфекционных заболеваний. У собак он также показан при глазных инфекциях из-за вторичных бактериальных осложнений от раскалывания.
Основные преимущества:
Эффективность широкого спектра в отношении как грамположительных, так и грамотрицательных организмов, включая псевдомоны aeruginsa. Комбинированный антибактериальный эффект окситетрациклина гидрохлорида и полимиксина в является, по крайней мере, добавкой, и во многих случаях происходит фактическое synergistic действие.
Доставка:
Доставка ежедневно из склада с турецкой почтой Все товары поставляются с номером отслеживания Наши пакеты прибывают в США и Канаду в течение 10 дней, европейские страны в течение 7 дней.
Характеристики
- Target животное
- Собак и кошек
Pfizer Labs
TERRAMYCINoxytetracyclinehydrochlorideCAPSULES
FULL PRESCRIBING INFORMATION: CONTENTS*
- TERRAMYCIN DESCRIPTION
- ACTIONS
- INDICATIONS
- TERRAMYCIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TERRAMYCIN ADVERSE REACTIONS
- TERRAMYCIN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
TERRAMYCIN DESCRIPTION
Oxytetracycline is a product of the metabolism of Streptomyces rimosus and is one of the family of tetracycline antibiotics. A 1 percent solution in water is acidic (pH about 2.5). Its potency is affected in solutions more acid than pH 2 and it is rapidly destroyed by alkali hydroxides.
Oxytetracycline diffuses readily through the placenta into the fetal circulation, into the pleural fluid and, under some circumstances, into the cerebrospinal fluid. It appears to be concentrated in the hepatic system and excreted in the bile, so that it appears in the feces, as well as in the urine, in a biologically active form.
Inert ingredients in the formulation are: glucosamine hydrochloride; hard gelatin capsules (which may contain Red 3, Yellow 10 and other inert ingredients); magnesium stearate; sodium lauryl sulfate; starch.
ACTIONS
Oxytetracycline is primarily bacteriostatic and is thought to exert its antimicrobial effect by the inhibition of protein synthesis. Oxytetracycline is active against a wide range of gram-negative and gram-positive organisms.
The drugs in the tetracycline class have closely similar antimicrobial spectra, and cross resistance among them is common. Microorganisms may be considered susceptible if the M.I.C. (minimum inhibitory concentration) is not more than 4.0 mcg/ml and intermediate if the M.I.C. is 4.0 to 12.5 mcg/ml.
Susceptibility plate testing: A tetracycline disc may be used to determine microbial susceptibility to drugs in the tetracycline class. If the Kirby-Bauer method of disc susceptibility testing is used, a 30 mcg tetracycline disc should give a zone of at least 19 mm when tested against a tetracycline-susceptible bacterial strain.
Tetracyclines are readily absorbed and are bound to plasma proteins in varying degree. They are concentrated by the liver in the bile, and excreted in the urine and feces at high concentrations and in a biologically active form.
INDICATIONS
Oxytetracycline is indicated in infections caused by the following microorganisms:
- Rickettsiae (Rocky Mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsialpox and tick fevers),
- Mycoplasma pneumoniae (PPLO, Eaton Agent),
- Agents of psittacosis and ornithosis,
- Agents of lymphogranuloma venereum and granuloma inguinale,
- The spirochetal agent of relapsing fever (Borrelia recurrentis).
The following gram negative microorganisms:
- Haemophilus ducreyi (chancroid),
- Pasteurella pestis, and Pasteurella tularensis,
- Bartonella bacilliformis,
- Bacteroides species,
- Vibrio comma and Vibrio fetus,
- Brucella species (in conjunction with streptomycin).
Because many strains of the following groups of microorganisms have been shown to be resistant to tetracyclines, culture and susceptibility testing are recommended.
Oxytetracycline is indicated for treatment of infections caused by the following gram-negative microorganisms, when bacteriologic testing indicates appropriate susceptibility to the drug:
- Escherichia coli,
- Enterobacter aerogenes (formerly Aerobacter aerogenes),
- Shigella species,
- Mima species and Herellea species,
- Haemophilus influenzae (respiratory infections),
- Klebsiella species (respiratory and urinary infections).
Oxytetracycline is indicated for treatment of infections caused by the following gram-positive microorganisms when bacteriologic testing indicates appropriate susceptibility to the drug:
Streptococcus species:
Up to 44 percent of strains of Streptococcus pyogenes and 74 percent of Streptococcus faecalis have been found to be resistant to tetracycline drugs. Therefore, tetracyclines should not be used for streptococcal disease unless the organism has been demonstrated to be sensitive.
For upper respiratory infections due to Group A beta-hemolytic streptococci, penicillin is the usual drug of choice, including prophylaxis of rheumatic fever.
Diplococcus pneumoniae,
Staphylococcus aureus, skin and soft-tissue infections. Oxytetracycline is not the drug of choice in the treatment of any type of staphylococcal infections.
When penicillin is contraindicated, tetracyclines are alternative drugs in the treatment of infections due to:
- Neisseria gonorrhoeae,
- Treponema pallidum and Treponema pertenue (syphilis and yaws),
- Listeria monocytogenes,
- Clostridium species,
- Bacillus anthracis,
- Fusobacterium fusiforme (Vincent’s infection),
- Actinomyces species.
In acute intestinal amebiasis, the tetracyclines may be a useful adjunct to amebicides.
In severe acne, the tetracyclines may be useful adjunctive therapy.
Tetracyclines are indicated in the treatment of trachoma, although the infectious agent is not always eliminated, as judged by immunofluorescence.
Inclusion conjunctivitis may be treated with oral tetracyclines or with a combination of oral and topical agents.
TERRAMYCIN CONTRAINDICATIONS
This drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
WARNINGS
THE USE OF DRUGS OF THE TETRACYCLINE CLASS DURING TOOTH DEVELOPMENT (LAST HALF OF PREGNANCY, INFANCY, AND CHILDHOOD TO THE AGE OF 8 YEARS) MAY CAUSE PERMANENT DISCOLORATION OF THE TEETH (YELLOW-GRAY-BROWN). This adverse reaction is more common during long term use of the drugs but has been observed following repeated short term courses. Enamel hypoplasia has also been reported. TETRACYCLINE DRUGS, THEREFORE, SHOULD NOT BE USED IN THIS AGE GROUP UNLESS OTHER DRUGS ARE NOT LIKELY TO BE EFFECTIVE OR ARE CONTRAINDICATED.
If renal impairment exists, even usual oral or parenteral doses may lead to excessive systemic accumulation of the drug and possible liver toxicity. Under such conditions, lower than usual total doses are indicated and, if therapy is prolonged, serum level determinations of the drug may be advisable.
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Patients apt to be exposed to direct sunlight or ultraviolet light should be advised that this reaction can occur with tetracycline drugs, and treatment should be discontinued at the first evidence of skin erythema.
The antianabolic action of the tetracyclines may cause an increase in BUN. While this is not a problem in those with normal renal function, in patients with significantly impaired function, higher serum levels of tetracycline may lead to azotemia, hyperphosphatemia, and acidosis.
Usage in pregnancy: (See above WARNINGS about use during tooth development.)
Results of animal studies indicate that tetracyclines cross the placenta, are found in fetal tissues and can have toxic effects on the developing fetus (often related to retardation of skeletal development). Evidence of embryotoxicity has also been noted in animals treated early in pregnancy.
Usage in newborns, infants, and children. (See above WARNINGS about use during tooth development.)
All tetracyclines form a stable calcium complex in any bone forming tissue. A decrease in the fibula growth rate has been observed in prematures given oral tetracycline in doses of 25 mg/kg every 6 hours. This reaction was shown to be reversible when the drug was discontinued.
Tetracyclines are present in the milk of lactating women who are taking a drug in this class.
PRECAUTIONS
As with other antibiotic preparations, use of this drug may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, the antibiotic should be discontinued and appropriate therapy instituted.
In venereal diseases when coexistent syphilis is suspected, a dark field examination should be done before treatment is started and the blood serology repeated monthly for at least 4 months.
Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage.
In long term therapy, periodic laboratory evaluation of organ systems, including hematopoietic, renal and hepatic studies should be performed.
All infections due to Group A beta-hemolytic streptococci should be treated for at least 10 days.
Since bacteriostatic drugs may interfere with the bactericidal action of penicillin, it is advisable to avoid giving tetracycline in conjunction with penicillin.
TERRAMYCIN ADVERSE REACTIONS
Gastrointestinal: anorexia, nausea, vomiting, diarrhea, glossitis, dysphagia, enterocolitis, and inflammatory lesions (with monilial overgrowth) in the anogenital region. These reactions have been caused by both the oral and parenteral administration of tetracyclines. Rare instances of esophagitis and esophageal ulcerations have been reported in patients receiving capsule and tablet forms of drugs in the tetracycline class. Most of these patients took medications immediately before going to bed. (See DOSAGE AND ADMINISTRATION.)
Skin: maculopapular and erythematous rashes. Exfoliative dermatitis has been reported but is uncommon. Photosensitivity is discussed above. (See WARNINGS.)
Renal toxicity: Rise in BUN has been reported and is apparently dose related. (See WARNINGS.)
Hypersensitivity reactions: Urticaria, angioneurotic edema, anaphylaxis, anaphylactoid purpura, pericarditis and exacerbation of systemic lupus erythematosus.
Bulging fontanels in infants and benign intracranial hypertension in adults have been reported in individuals receiving full therapeutic dosages. These conditions disappeared rapidly when the drug was discontinued.
Blood: Hemolytic anemia, thrombocytopenia, neutropenia and eosinophilia have been reported.
When given over prolonged periods, tetracyclines have been reported to produce brown-black microscopic discoloration of thyroid glands. No abnormalities of thyroid function studies are known to occur.
TERRAMYCIN DOSAGE AND ADMINISTRATION
Adults: Usual daily dose, 1–2 g divided in four equal doses, depending on the severity of the infection.
For children above eight years of age: Usual daily dose, 10–20 mg per pound (25–50 mg/kg) of body weight divided in four equal doses.
Therapy should be continued for at least 24–48 hours after symptoms and fever have subsided.
For treatment of brucellosis, 500 mg oxytetracycline four times daily for 3 weeks should be accompanied by streptomycin, 1 gram intramuscularly twice daily the first week, and once daily the second week.
For treatment of uncomplicated gonorrhea, when penicillin is contraindicated, tetracycline may be used for the treatment of both males and females in the following divided dosage schedule: 1.5 grams initially followed by 0.5 gram q.i.d. for a total of 9.0 grams.
For treatment of syphilis, a total of 30–40 grams in equally divided doses over a period of 10–15 days should be given. Close follow-up, including laboratory tests, is recommended.
Administration of adequate amounts of fluid along with capsule and tablet forms of drugs in the tetracycline class is recommended to wash down the drugs and reduce the risk of esophageal irritation and ulceration. (See ADVERSE REACTIONS.)
Concomitant therapy: Antacids containing aluminum, calcium, or magnesium impair absorption and should not be given to patients taking oral tetracyclines.
Food and some dairy products also interfere with absorption. Oral forms of tetracyclines should be given 1 hour before or 2 hours after meals. Pediatric oral dosage forms should not be given with milk formulas and should be given at least 1 hour prior to feeding.
In patients with renal impairment (See WARNINGS.) Total dosage should be decreased by reduction of recommended individual doses and/or by extending time intervals between doses.
In the treatment of streptococcal infections, a therapeutic dose of oxytetracycline should be administered for at least 10 days.
HOW SUPPLIED
Terramycin (oxytetracycline HCl) Capsules are available as opaque, yellow, hard gelatin capsules which contain oxytetracycline HCl equivalent to 250 mg of oxytetracycline, and glucosamine hydrochloride: bottles of 100 (NDC 0069-0730-66), 500 (NDC 0069-0730-73).
Rx only
69-0755-32-7
March 1987
Terramycin
Oxytetracycline Hydrochloride CAPSULE
Product Information |
|||
|---|---|---|---|
| Product Type | Human prescription drug label | Item Code (Source) | NDC:0069-0730 |
| Route of Administration | ORAL | DEA Schedule |
Active Ingredient/Active Moiety |
||
|---|---|---|
| Ingredient Name | Basis of Strength | Strength |
| Oxytetracycline Hydrochloride OXYTETRACYCLINE ANHYDROUS | 250 mg |
Inactive Ingredients |
|
|---|---|
| Ingredient Name | Strength |
| GLUCOSAMINE HYDROCHLORIDE | |
| hard gelatin capsules | |
| Red 3 | |
| Yellow 10 | |
| MAGNESIUM STEARATE | |
| SODIUM LAURYL SULFATE | |
| starch |
Product Characteristics |
|||
|---|---|---|---|
| Color | Size | Imprint Code | Shape |
| YELLOW (Opaque Yellow) | 22 mm | Terramycin;Pfizer;073 | CAPSULE |
Packaging |
||||
|---|---|---|---|---|
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC:0069-0730-66 | 100 in 1 BOTTLE | ||
| 2 | NDC:0069-0730-73 | 500 in 1 BOTTLE |